Introduction | dynamic surface patterns
It is well-known that cells respond to the patterns on surfaces by adapting via differentiation. Cell-biomaterial interaction has long been studied using static surface patterns. However static patterns do not recapitulate the physiological conditions, since, inside the body, cells experience a continuously-changing environment. Especially macrophages are exposed to ever-changing surfaces in vivo due to their ability to migrate between tissues. Therefore, studying macrophage-material interactions using dynamic surface topographies is the only way to unambiguously elucidate which cellular properties correlate with ever-changing surfaces in physiologically-relevant conditions. Dynamic surface patterns can help us understand hitherto unnoticed cell behavior that has impact on refining the implant design strategies and tissue repair therapies.
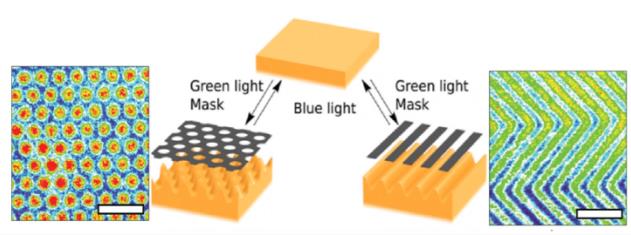 Figure 1. The switch between different surface patterns under influence of green and blue light. The scale bar represents 100 μm. (Hendrikx et al., 2014)
Figure 1. The switch between different surface patterns under influence of green and blue light. The scale bar represents 100 μm. (Hendrikx et al., 2014)
We hypothesize that macrophages respond to dynamic surface patterns and change their phenotype (M0/M1/M2) accordingly. The versatility of macrophages originates from their ability to shift between these phenotypes. We are particularly interested in modeling the macrophages in retinal blood flow, where they experience both fluid flow and surface pattern and stiffness changes when changing their location.
For the dynamic surface patterns, we use liquid crystal networks (LCN) which can dynamically change shape upon light illumination (Figure 1). For the fluidic conditions, we use microfluidic chips. In the setting, LCN are integrated in microfluidic chips where the cells can be cultured conveniently (Figure 2).
Project | Shear stress and surface pattern dependent macrophage polarization
In this project, we will strive for understanding how the macrophages transform depending on shear stress changes and dynamic surface patterns. We monitor the cells via PCR and imaging.
We developed a dynamic surface-on-chip platform where the cells can experience both dynamic changes in the surface that they attach and a flow rate on top of them to mimic the conditions in retinal blood vessels. We want to improve this platform and use it for investigating macrophage polarization.
 Figure 2 . Left An integrated microfluidic chip consisting of PDMS with LCN layer. Fluid flow is applied using a syringe pump and the waste is collected in a beaker. Right Macrophages grown on a LCN surface are stained with DAPI (nuclei) and phalloidin (cytoskeleton).
Figure 2 . Left An integrated microfluidic chip consisting of PDMS with LCN layer. Fluid flow is applied using a syringe pump and the waste is collected in a beaker. Right Macrophages grown on a LCN surface are stained with DAPI (nuclei) and phalloidin (cytoskeleton).
Collaboration | In this project, we collaborate with Prof. Albert Schenning at Chemical Engineering and Chemistry Department.
Goal | milestones and achievements
The goals of this project are:
- To grow THP-1 macrophages and native macrophages in a microfluidic chip, where shear stress and dynamic surface patterns are applied.
- Investigate biomarkers and use them for identifying the effect of the cues on the macrophage polarization.
References
- Hendrikx, M., Ter Schiphorst, J., van Heeswijk, E. P., Koçer, G., Knie, C., Bléger, D., ... & Schenning, A. P. (2018). Re‐and Preconfigurable Multistable Visible Light Responsive Surface Topographies. Small, 14(50), 1803274.
- Mita, Y., Dobashi, K., Nakazawa, T., & Mori, M. (2001). Mechanical fluid flow and surfactant-TA influence activation of macrophages. In Vitro Cellular & Developmental Biology-Animal, 37(5), 270-274.
- Koçer, G., Ter Schiphorst, J., Hendrikx, M., Kassa, H. G., Leclère, P., Schenning, A. P., & Jonkheijm, P. (2017). Light‐Responsive Hierarchically Structured Liquid Crystal Polymer Networks for Harnessing Cell Adhesion and Migration. Advanced materials, 29(27), 1606407.

